This topic has been on my mind for some time. As the supplement industry has expanded to become a billion dollar industry, I have seen the market flooded with poor quality (not necessarily cheap) supplements. Unlike prescription drugs, vitamins and supplements are not subject to regular testing of any kind. Many companies source raw material for manufacture from China or India. Interestingly, in China there is a huge market for pure, high quality supplements from Australia. The Chinese consumer is wary of the quality of the local product, as evidenced the recent baby milk formula scandal.
The PAN Pharmaceutical Controversy
In 2003 the Therapeutic Goods Administration (TGA) recalled nearly 1,600 products manufactured by PAN Pharmaceuticals in Australia. The conspiracy theorists believe that this was collusion between the TGA and the pharmaceutical industry to discredit the supplement industry. Interestingly, although the company was fined a total of $13million, eventually the government paid out $112.5million to the owner of the company and his creditors. The payout was due to the mistakes that were made by the TGA during the investigation. It also resulted in an inquiry immediately after (The Expert Committee on Complimentary Medicines in the Health System). There were dubious manufacturing practices in the company, which identified:
- substitution of ingredients
- manipulation of quality control test results
- substandard manufacturing practices
- failure to test raw ingredients
- failure to clean manufacturing equipment between batches
- cross-contamination of products
- imported raw materials found not to have passed appropriate quarantine checks
- overall poor hygiene and sanitation
- failure to test raw materials for potential pathogens prior to manufacture
The above list is what we, as the consumer, would expect a supplement company NOT to be doing in supplying products to us. The reality is that there are very few reputable companies that supply high quality supplements. As a practitioner I need to be confident that the supplements that I am supplying to my patients are of high quality and that what is on the label is indeed the product that is in the bottle. Reputable manufacturers will show you their data with independently verified certificate of analysis of their product.
I hear reports of expired supplements being relabeled and sold on the internet. I have heard of rejected batches of supplements that do not meet quality control specifications being sold as well. A company representative recently told me of a practice in an overseas country where empty bottles of supplements were being refilled with capsules or tablets of dubious quality, resealed and then sold on to unsuspecting consumers with the original label.
Counterfeit medicines and supplements are big business for rogue operators. For example, in May 2014, an Interpol coordinated operation in Britain arrested 237 people worldwide in a 10-day crackdown on fake drugs, resulting in the seizure of counterfeit and unlicensed medicines worth $31.4 million. The crackdown also targeted 10,603 websites, leading them to be closed down or suspended. The Head of Enforcement, at the Medicines and Healthcare Products Regulatory Agency (MHRA) said “The medicines recovered during these raids were being held in appalling conditions, such as a dirty old building with broken windows, with medicines lying on the floor in bin bags.” “Criminals involved in the illegal supply of medicines through the Internet aren’t interested in your health; they are interested in your money.” India was the source of 72% of the illicit medicines seized in Britain, while China accounted for 11 percent.
What Goes Into a Good Quality Supplement?
Consumers would expect that the supplement that they are purchasing is:
- Free of contamination
- Contains the ingredients on the label in the specified amounts
- Contains an active form of the ingredient (whether it is a herb or vitamin)
- Dosage is safe and efficacious
- Free of allergens and excipients that consumers may react to
- Well absorbed (tablet breaks apart quickly so that the ingredients can be absorbed)
- Trackable from source of ingredients to quality of manufacture
- Produced in a Good Manufacturing Practice (GMP) facility
- Independent testing of raw ingredients and the final product
- Stability data showing that product ingredients are viable well past the stated expiry date
Below is an example of one manufacturers process flow for the production of a herbal supplement. Beginning with buying from a sustainable source (wherever possible), to the quality control of the final product. There is a lot more involved than just buying in the raw material and putting it into a capsule, which probably occurs more often than we would like to believe.
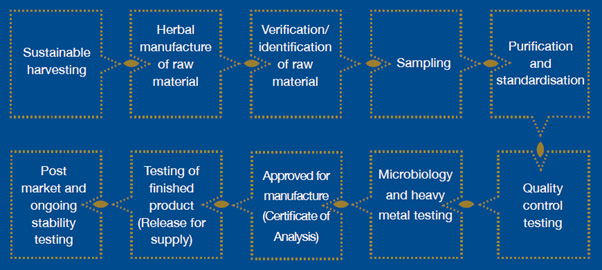
Testing Raw Materials
Any reputable GMP manufacturing facility should check each of the individual raw materials in their product prior to production. The testing should ensure where appropriate:
- Product conforms to required materiel specification (chemical composition, water content, strength or potency, etc)
- Check for heavy metal, pesticide or solvent contamination
- Herbal constituents are the correct species, plant parts and active constituents
- No fungus, mould, aflatoxins or other microbial contamination
- No rancidity of the product
- Check for GMOs and other environmental pollutants
Setting up a proper GMP production facility is expensive, there are no short cuts. Practitioner products occasionally become unavailable, simply because the manufacturer is unable to source raw material of suitable quality. Although this is an inconvenience, it should also be reassuring that these manufacturers will not accept substandard ingredients in their products. All this needs to be factored into the price of the product. So here is the problem that reputable supplement manufacturers have. They spend all this money on ensuring that their product is of superior quality and put their product on the shelf. Often consumers find another product that is a few dollars cheaper, but possibly unknowingly been manufactured in someone’s garage or from questionable raw materials, they will go with the cheaper product. Unknowingly buying a product that is of poor quality at best, useless or harmful at worst. If they go online, there is no guarantee the supplement was sourced from the original manufacturer, even if it has a reputable company’s logo on the bottle.
Next time you pick up a supplement, instead of considering the price, it is more important to consider whether the product is what you are paying for. How confident are you that what is on the label of the bottle is actually in the capsule or tablet you are about to consume? If you are about to give it to your child, then you would want to be sure that you are giving a good quality supplement, rather than expensive confectionary or something nasty.
Multivitamin Mineral Supplements
In 2008 the FDA in the US released testing results on 324 multivitamins specifically formulated for women and children that were purchased over the internet. The results indicated that almost all vitamins produced for consumption by women and children contained trace amounts of lead. Although the FDA did not take a position on whether the levels of lead in these vitamins posed a health hazard, five samples would have provided exposures that exceeded 4 µg/day, the highest being 8.97 µg/day. As pointed out by a presentation at the MINDD conference in May 2015 on the issue of lead, there is no known safe level of lead exposure.

In children’s supplements care must be taken to consider the added colours, flavours and other excipients, which sensitive children may react to. Many of the “gummy bear” supplements are high in sugar and like muesli bars should be relocated to the confectionary aisle.
Fish Oil Supplements – Here’s some fishy business
The three main fish oil types available are natural triglycerides (TG), ethyl esters (EE) and re-esterified triglycerides (rTG).
TG – fatty acids in fish oils are naturally found in triglyceride form
EE – Many fish oils are processed by reacting free fatty acids with ethanol to form ethyl esters. This process is followed by molecular distillation to remove impurities, short chain fatty acids and saturated fats, allowing for higher concentration of EPA/ DHA in fish oil
rTG -this takes oils from the above step (EE) and converts it back to triglycerides. This process increases the total triglyceride concentration of oil from 30% to 60%.
So what? The problem is that while TG and rTG can be easily digested and absorbed by the small intestine, the EE form is more resistant to breakdown than TG. Of the fish oil products on the market, a high number are produced in EE form. It is no doubt due to certain manufacturers seeking to minimise production costs, as reconverting oils back to TG form from EE (as for rTG products) can increase costs dramatically. A recent study found the relative bioavailability of different types of fish oils to be: rTG (124%), FFA/TG (91%), EE (73%). Therefore there is a big difference in how we are able to absorb different forms of fish oils. In turn there will be a big difference in how effective a fish oil product is.
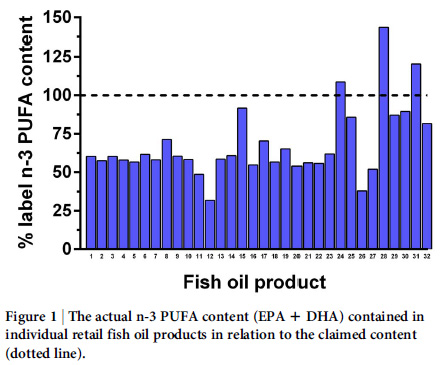
A recent NZ fish oil supplement study found:
Only 3 of 32 fish oil supplements contained quantities of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that were equal or higher than the content on the label, with most products tested (69%) containing only 67% of EPA/DHA. Which of these 32 products would you prefer to be paying for and consuming or giving to your children?
Oxidation (rancidity) can be detected by measuring the peroxide value (PV) and the anisidine value (AV). PV reflects the primary oxidation products (lipid peroxides) while AV reflects secondary oxidation products (aldehydes and ketones). Together, these parameters are used to estimate the total oxidation value (Totox).
The vast majority of omega-3 supplements exceeded recommended levels of oxidation markers. 83% products exceeded the recommended PV levels, 25% exceeded AV thresholds, and 50% exceeded recommended Totox levels. Only 8% met the international recommendations, not exceeding any of these indices for rancidity!
The discrepancy between actual and labelled omega-3 content is consistent with a study of retail fish oils sold in South Africa. This older international survey where products were purchased in Canada, USA and UK; found that more than half of the omega-3 oil products surveyed contained less than 90% of the omega-3 claimed on the label. The authors of the paper state that it is interesting that 17 products listed exactly the same concentration of omega-3 (180 mg EPA and 120 mg DHA per gram of oil). Such striking uniformity suggests that the companies selling these products have relied on data provided by extractors who supply fish oil to multiple brands. Thus, the labelled composition may not reflect the final n-3 PUFA content of product, which may have changed due to oxidation during transport, encapsulation, packing, and storage.
Evidence from animal studies show that large doses of oxidised lipids may cause organ toxicity, growth retardation, and accelerated atherosclerosis.
Only one relatively short study has compared the effects of oxidised fish oil in humans, observing no evidence of acute oxidative toxicity. However, the effects of long-term exposure to oxidised oils (particularly on markers associated with atherosclerosis) have not been studied. The oxidative state of fish oils used in clinical trials has never been reported, so the possible effects of oxidation on trial outcomes are unknown. It is possible that the conflicting and often disappointing results in clinical trials may have resulted from the use of highly oxidised omega-3 supplements.
Two products that were for practitioner only dispensing also had excess oxidation products, and though one was labelled with the peroxide value (PV), the true PV exceeded the label by more than four-fold! With that in mind it was a relief to find that the product that I stock in my dispensary exceeded the EPA and DHA content, and was well within international standards for oxidation markers.
Herbal Supplements – Not all herbs are the same
Sufficiently pure, good quality herbal ingredients for manufacture can be very expensive. Some less-than-honest suppliers are economically motivated to find ways to “trick the system”, engaging in more and more devious ways to adulterate, spike and substitute botanical ingredients. This results in herbal medicine raw materials on the market with questionable efficacy, and potentially dangerous outcomes.
Using the herb Ginkgo as an example, three Ginkgo aglycone constituents are used to check the quality and efficacy of the herb; quercetin, kaempferol, and isorhamnetin. Low-cost, sub-standard Ginkgo extracts are frequently spiked with one of these three constituents (usually quercetin or rutin), or substituted with low-cost adulterants from less active parts of the Ginkgo biloba tree. Adulteration with other plant species including Sophoro loponica is done to increase total aglycone content, which without sophisticated analytical procedures, cannot be detected.
Apart from product labels often not reflecting the contents of the herbal capsule or tablet. There are more serious issues to consider:
- Fungal infection and aflatoxin B1 contamination of herbal raw materials
- Accumulation of toxic industrial effluents in the soil is continuously increasing due to extensive pollution of the environment. Plants are sensitive to environmental growing conditions and they accumulate environmental heavy metals throughout the plant
- There are reports of Traditional Chinese Medicine supplements containing antibiotics or other pharmaceutical drugs
Probiotics – Do you trust what’s inside the supplement?
LabDoor is a company in the US that tests supplements and probiotics for their quality, and compares label claims with their test results. Some of their test results have found:
- Gummy and chewable probiotic supplements (5/30) recording 92% less probiotic bacteria than standard formulations. Also consisted of fewer probiotic species (25/30).
- Total viable bacteria ranged from -99% to +2400% vs. the products’ stated label claims.
Probiotic Label Claims
In one study, worldwide probiotic culture suppliers were contacted and asked to submit probiotic strain samples for analysis. These large probiotic suppliers supply probiotics to commercial manufacturers of probiotic supplements. More than 28% of the commercial cultures intended for human and/or animal probiotic use were found to be misidentified at the genus or species level. Misidentified strains were supplied by 10 out of the 26 participating companies. This is a concern that the suppliers of probiotic cultures for probiotic supplements did not have their probiotic strains correctly identified. For this reason the more reputable manufacturers will independently test the probiotic cultures via DNA “fingerprinting” to ensure they have received the correct strains prior to manufacture.
Gluten in Probiotics
In a US study reported in May 2015, 22 of the most popular brands of probiotics on the national market were evaluated for gluten. They found that more than half (53%) of the probiotics labelled as gluten-free contained the protein composite (gluten), and some of these (13%) contained more than 20 ppm, which exceeds the FDA threshold for the gluten-free labelling of food.
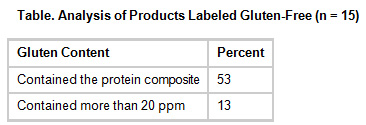
Seven (31%) of the 22 probiotics tested, not labelled gluten-free, of these, four tested positive for gluten, and two contained more than 20 ppm. It should be kept in mind that taking multiple doses of probiotics throughout the day can have a considerable additive effect for gluten. Those feeling unwell while taking probiotics may unknowingly be reacting to the gluten content within the product.
Buying from internet retailers – beware
- If you find a name brand online that is markedly cheaper than elsewhere, question why?
- Product may be expired and relabelled, or counterfeit
- Can you track the source of the item? Was it sourced directly from the manufacturer? Major third party retailers are not responsible for the quality of the items processed through them
- If the manufacturer does not sell to the retailer, how are they getting the product?
References
Medicines Worth USD 31 Million Seized in Global Crackdown. Medscape. May 22, 2014.
A Vitamin a day may do more harm than good. ConsumerLab.com report finds unexpected nutrient levels, contamination.https://www.nbcnews.com/health/health-news/vitamin-day-may-do-more-harm-good-flna1C9471899
Survey Data on Lead in Women’s and Children’s Vitamins.http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm115941.htm
Analysis of over the counter dietary supplements. Green GA, et al. Clin J Sport Med. 2001 Oct;11(4):254-9.
Fungal contamination of raw materials of some herbal drugs and recommendation of Cinnamomum camphora oil as herbal fungitoxicant.Singh P, et al. Microb Ecol. 2008 Oct;56(3):555-60.
Investigation of heavy metals in frequently utilized medicinal plants collected from environmentally diverse locations of north western India.Kulhari A, et al. 2013 Dec 17;2:676.
Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Albert BB, et al. 2015 Jan 21;5:7928.
Bioavailability of marine n-3 fatty acid formulations.Dyerberg J, et al.Prostaglandins Leukot Essent Fatty Acids. 2010 Sep;83(3):137-41.
Huys,G.,M.Vancanneyt,et al. (2006). Accuracy of species identity of commercial bacterial cultures intended for probiotic or nutritional use. Research in Microbiology 157(9): 803-810.
‘Gluten-Free’ Often a Misnomer. Medscape. May 17, 2015



